Jennifer A. Cruce
Introduction
Yap State comprises approximately 134 islands and 11 atolls located within the Federated States of Micronesia (FSM) (Fig. 1a) and stretches from 6 to 10 degrees North Latitude and 137 to 148 degrees East Longitude. Of these islands (from the main island of Yap Proper to the neighbouring island atolls) 22 are inhabited by the Yapese people, who continue to closely follow traditional culture. Turtles are an integral part of many aspects of Yapese life, and this is readily apparent, predominantly in the outer islands. Four species of turtles have been found nesting and foraging within Yap State. Sightings of leatherback turtles (Dermochelys coriacea) have been reported in pelagic waters (McCoy, 1974) and olive ridley turtles (Lepidochelys olivacea) have been sighted swimming outside the reef of Yap Proper (Falanruw 1975). One olive ridley turtle was caught alive by a local fisherman in the water near Rull Municipality, Yap, on July 18, 2007 and subsequently PIT tagged and released. Green turtles (Chelonia mydas) are the most common species nesting in the neighbouring islands and hawksbill turtles (Eretmochelys imbricata) are often seen foraging in Yap mainland and neighbouring island reefs.
Ulithi Atoll, located approximately 115 miles northeast of Yap Proper, is home to several ‘Turtle Islands’ (Fig. 1b), of which five are identified as significant green turtle nesting sites by the local people. These uninhabited islands include a cluster of three islands: Loosiep, Bulbul and Yeew, located approximately 12 kilometres (km) south-southeast of Falalop, and a cluster of two islands: Gielop and Iar, which may be among the largest green turtle rookeries in Micronesia (Kolinski, 1992). Turtles nesting on or mating near these islands have traditionally been hunted for their meat and eggs (Lessa, 1983). Green turtle meat and eggs have historically been an important source of protein for inhabitants of the outer islands; whereas hawksbill turtles are primarily sought for their shell and seldom used as a food source (McCoy, 1981). Falalop accommodates one of two high schools on Yap’s outer islands and as a result supports the largest population in Ulithi Atoll. Since areas around the island of Falalop lack good fishing grounds (Ruddle, 1996), local people seek green turtles, which provide more food with less effort than fishing. Nesting populations in the past have been managed and inadvertently conserved due to cultural harvesting limitations put in place by the people of the chief island, Mogmog, for all other islands within Ulithi (Lessa, 1983). Traditionally, these restrictions dictate that all turtles caught within Ulithi Atoll must be taken to Mogmog for ritualistic slaughter and division of the turtle meat (Lessa, 1983). Turtle take in Ulithi has not been assessed or quantified.
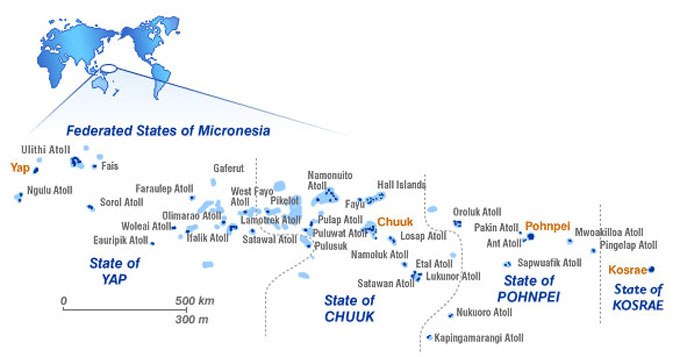
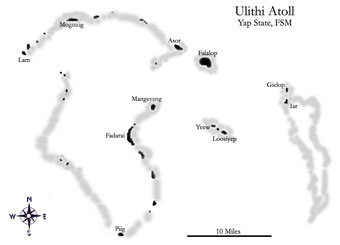

Fig. 1. (a) Regional map of Federated States of Micronesia depicting Yap State (mainland), Ulithi Atoll and neighbouring outer islands.
(b) Illustration of Ulithi Atoll depicting ‘turtle islands’ located southeast of Falalop.
(c) Photo of Yeew and Bulbul Islands with Loosiep Island in the background.
Nesting turtle monitoring fieldwork has been conducted in past and recent years on Gielop Island, Ulithi Atoll. Yap State Marine Resources Management Division (MRMD) tagged and collected life history data of nesting turtles on Gielop Island from May to August 1991; however, subsequent tagging projects were not pursued on Gielop due to limited resources and restricted access to the island. A second tagging and monitoring project was initiated through MRMD from June to August 2005 on Gielop and Iar Islands. In an effort to establish a long-term monitoring programme a second and third consecutive year of tagging was conducted on Gielop Island in 2006 and 2007 (June to August). The number of individual nesting green turtles tagged on Gielop Island between 2005 and 2007 totalled 888. Based on initial findings, and according to a NOAA Southwest Fisheries Science Center (SWFSC) Marine Turtle Research Program (MTRP) progress summary of tissue samples collected during the 2005 Gielop nesting season, a new haplotype (CMP32) was identified, which has not been found in any other turtle nesting population. A haplotype or haploid genotype is a set of closely linked genetic markers present on one chromosome which tend to be inherited together. This finding is significant in identifying a local nesting population unique to this area.
Island biodiversity and threats
The uninhabited islands of Ulithi are home to a number of native bird species, including red-footed boobies, various species of terns (Anous sp.) and the Great Frigatebird (Fregata major), the ghost crab (Ocypode cordimana), hermit crab (Coenobita compressus), coconut crab (Birgus latro), the endemic giant Micronesian gecko (Perochirus scuttelatus) and a newly discovered endemic species of blind snake (Ramphotyphlops sp. nov.). An introduced monitor lizard has populated the island of Loosiep (Fig. 1c), which has also been identified as an important green turtle nesting beach. Monitor lizards are not currently found on the other four turtle islands.
This species is believed to be the mangrove monitor lizard (Varanus indicus) (Fig. 2) and is the only species of monitor lizard found throughout Yap’s islands (Fisher, 1948). The mangrove monitor lizard was introduced to many of the islands throughout Micronesia during the Japanese occupation in the early twentieth century to be used as a food source and pest control (Lever, 2003). Local inhabitants of Yap do not utilise monitor lizards as a resource and consider them a threat to native nesting bird and turtle populations. Monitor lizards are known to be opportunistic predators and will prey on bird and turtle nests (McCoid & Wittman 1993). Local landowners throughout Yap have attempted to control and even eradicate lizard populations with little to no success. Currently, there have been no studies conducted on monitor lizard population on the islands of Yap; therefore the affect, if any, monitor lizards have had on local turtle nesting populations has not been quantified or assessed.
The mangrove monitor lizard ranges from 75 to 120 centimetres in length and is darkly coloured with small yellow spots (Fig. 2). Females tend to be reasonably smaller than the males. Their diet consists of insects, crabs, small mammals, lizards, lizard eggs, birds and bird eggs (McCoid & Wittman, 1993). A trait unique to V. indicus is the ability to extend the lower jaw to eat larger prey. Monitors are known to feed on turtle eggs and hatchlings (Bennett, 2002) as they adapt to their environment and the food resources available to them. When food is abundant females will nest year round laying multiple small clutches (Bennett, 2002). On atoll islands located in the Mortlocks, which is the furthest, most westerly island of Chuuk State, FSM (east of Yap), monitors were observed in trees, on the forest floor, and in the open sand (Buden, 2007). They are known to swim and dive, climb, leap between trees, and dig with great agility (Bennett, 2002).
After the introduction of monitor lizards to most Micronesian islands for biological control of creatures such as rats, beetles, and ants, the population grew significantly. The poisonous cane toad (also known as bullfrog or giant toad) (Bufus marinus) was then introduced to regulate the growing population. While monitor lizard numbers declined, the toad became a biological pest eliminating both harmful and beneficial invertebrates (ISSG 2007). The cane toad is not known to have been introduced to the turtle islands of Ulithi Atoll.
Studies specifically investigating what effect introduced monitor lizards have on turtle nesting beaches in Micronesia have not been conducted. Although they are known to prey on sea turtle eggs and hatchlings (ISSG 2007), no direct correlation to adverse effects on nesting turtle populations has been made. To address these questions and the concerns of island landowners, the 2008 nesting turtle monitoring project was located on Loosiep Island. The goal of the research conducted during the 2008 nesting season was to collect information on the nesting green turtle population by counting the number of turtles emerging, and assessing the internesting interval, reproductive success, health and population genetics, and to assess the effects of monitor lizard predation of turtle nests.
Study sites
Gielop is situated at N 9° 56.716', E 139° 54.45' and Loosiep at N 9° 55.09', E 139° 50.58' located approximately 15.4 km south and southeast of Falalop, Ulithi, Yap State, Federated States of Micronesia (Fig. 1). Gielop and Iar Island are situated at the northern tip of a separate coral reef system that is locally referred to as Meteral Atoll (Kolinski, 1992). Loosiep, Bulbul (N 9° 55.09', E 139° 50.58'), and Yeew (N 9° 55.09', E 139° 50.58') Islands form a separate reef system.
Nesting turtle population assessment
Estimating marine turtle nesting trends ideally requires a 20 to 25 year survey effort (Chaloupka et al., 2007). Consecutive years of nesting beach monitoring and tagging conducted on Gielop Island from 2005 to 2007 and on Loosiep Island in 2008 were based on an initial study conducted by MRMD in 1991. Methods used during the 1991 project were followed closely in an attempt to replicate the survey and design so that the results may be comparable (Richardson, 1999). These methods focused primarily on assessing the number of nesting turtles, number of nests, hatching success, tissue sampling for DNA analysis, and sampling turtles with carapace lesions or tumours. Track counts were not routinely conducted during these studies. As only four years (three of those consecutive and all during the latter portion of the nesting season) of night-time monitoring activities have been conducted on Gielop Island, this project is still in the early stages of developing a valid monitoring program. An accurate measurement of the nesting population requires conducting surveys throughout the entire season and a complete tagging census (Limpus et al., 2003). A total of 888 individual nesting green turtles and one nesting hawksbill turtle were tagged on Gielop Island between 2005 and 2007. In 2008, a total of 66 nesting green turtles and three male green turtles were tagged and assessed.
Identifying the peak of the nesting season is integral to understanding sea turtle population trends. Most turtle populations nest during a distinct time of year (Godley et al., 2001), with the peak of breeding for green turtles typically consistent between years, which is the case for the Ascension Island and Tortuguero nesting populations (Godley et al., 2001; Troeng & Rankin, 2005). On Raine Island, Australia, green turtles nest year-round and although there is considerable annual variability, the peak is December and January (Limpus et al., 2003). Yearly variations in the nesting peak could be explained by climate changes affecting temperatures of the nesting beach and El Nino Southern Oscillation events or suitability of foraging grounds affecting the health of reproductive females (Limpus et al., 2003; McGowan et al., 2008).
According to local fishermen, the turtle nesting season in Ulithi Atoll extends roughly from March to September. Based on data collected from 1991, May and June represent the months with the highest nesting activity although May appears to be the peak month of the nesting season. This project has routinely been conducted from May to August (1991) and June to August (2005 to 2007) so as to encounter the maximum number of turtles. The fieldwork on Loosiep Island in 2008 was carried out from April to July to include the peak nesting days of the season. However, nesting activities appeared generally consistent through the monitoring period with late May representing the highest nesting activity. Subsequent surveys should be conducted throughout the entire nesting season to make a more accurate prediction of the peak of the season.
Remigration and recapture data
Nesting turtles tagged on Gielop Island from 2005 to 2007 have not been observed returning to nest nor have tag returns been reported in Ulithi. Monitoring activities were not conducted on Gielop during the 2008 nesting season due to a temporary moratorium placed by the landowners restricting access to the island and instead were carried out on Loosiep Island located approximately 10 km southwest of Gielop. It is unknown whether turtles tagged from 2005 to 2007 returned during 2008; however, no tags were reported on turtles taken near Gielop by local fishermen during the 2008 season. In addition, turtles tagged from 2005 to 2007 were not seen returning to nest on Loosiep Island. Based on this information, remigration for this nesting population may be greater than 3 to 4 years. Tag loss, surveillance error, or incomplete seasonal monitoring could be contributing factors to the lack of remigration data (Richardson, 1999). It is also likely that the turtles nesting on Gielop do not utilise nesting beaches on Loosiep, Bulbul and Yeew, which may be confirmed though mitochondrial DNA analysis from tissue samples collected in 2008 or if the use of satellite telemetry becomes available during future surveys on Loosiep.
Recapture of 19 turtles tagged in Yap State between 1990 and 1992 was documented in 1995 (Kolinski, 1995). Recapture sites included the Philippines, Marshall Islands, Papua New Guinea and Yap Proper. Seven of the 19 turtles were originally tagged on Gielop Island in 1991. Of these seven, five were recovered in the Philippines, one in the Marshall Islands, and one on Yap Proper (Kolinski, 1995). Tag recovery or reporting has not been implemented in Yap State since 1995 nor has local take of turtles been assessed or quantified. Successive studies should include an organized tag recovery program throughout Yap State.
Satellite telemetry
Satellite telemetry has played an important role in the study of marine turtles worldwide. In August of 2005 a nesting green turtle was fitted with a satellite transmitter on Gielop Island. After spending five weeks in Ulithian waters, the turtle travelled westbound approximately 1,620 km to Tawitawi, Philippines, where she foraged for four months before transmission was lost. Because of successful tracking efforts in 2005, important migration data revealing resident feeding grounds for one Ulithian nesting green turtle created community and regional awareness. In August of 2006, six more nesting turtles were fitted with transmitters. Of these, four were tracked to the Philippines, one to Malaysia, and transmission was lost on the sixth turtle after being tracked for 39 days in Ulithi (Kolinski et al., 2007; in review). This information can be utilised in addressing local marine management issues concerning the conservation of turtles on an international and local level. By using metal flipper tags and satellite telemetry to study the movements of turtles in Yap, more can be learned about where post-nesting Yap turtle populations migrate and foraging areas may be determined.


Fig. 4. (a) First nesting green turtle fitted with a satellite transmitter on Gielop Island in 2005.
Fig. 4. (b) Field assistants attaching a satellite transmitter to a nesting green turtle on Gielop Island in 2007.
Observations of monitor lizards
Monitor lizards have been reported on Loosiep Island and historically on Bulbul and Yeew Islands by local landowners. During the 2008 fieldwork, monitor lizards were present on Loosiep but no evidence of monitors was observed on Bulbul or Yeew. Upon inspection of the three islands, biodiversity appeared to be notably affected on Loosiep. Coconut crabs, nesting birds, geckos and lizards were found on Bulbul and Yeew, whereas Loosiep was absent of many of these species. A total of 65 green turtle nests were observed on Loosiep in 2008, of which 28 were marked with wooden stakes. Of these 28, 82% (23) were predated by monitor lizards. The five that were not predated were located near the camp, which may have been a result of human activity. Nests were predated by monitor lizards within one to five days following egg deposition. It was also observed that several nests predated by monitors were subsequently dug by wild pigs. This finding warrants further investigation of the monitor lizard population on Loosiep, in addition to the implementation of a control or eradication programme.
Conclusions
Long-term research and conservation efforts on nesting sea turtles globally has led to the recovery of populations in Hawaii, Ascension Island, Tortuguero and the Great Barrier Reef (Balazs & Chaloupka, 2004; Broderick et al., 2006; Chaloupka & Limpus, 2001; Hays, 2004; Solow et al., 2002). In Tortuguero, information collected on nesting beaches provided a basis for regional policy decisions to be made that would improve protection of sea turtle nesting beaches (Troeng & Rankin, 2005). Hawaii’s conservation efforts since 1978 have yielded a substantial recovery of the resident sea turtle population (Balazs & Chaloupka 2004). Providing relevant information on population trends and size for traditional leaders and government agencies in Yap state is the first step in establishing a long-term conservation management plan. Locally, the foremost threat to sea turtles in Yap is from human consumption through the take of eggs and adult turtles. Green and hawksbill turtles are regularly taken from nesting beaches and nearby waters throughout Yap State. Currently there are two laws at the state and federal level regarding sea turtles; however, there is little to no enforcement of these laws throughout the FSM. Recovery of over-exploited and depleted turtle populations have been documented when appropriate protection measures have been applied (Chaloupka et al., 2007). Continued long-term monitoring of Loosiep and Gielop Islands and surrounding turtle islands nesting turtles may provide a more accurate estimation of the stability of the population, which in turn may lead to the enactment of policies and enforcement to prevent future exploitation of turtles and eggs.
In addition to long-term tagging and monitoring efforts on the turtle islands, a programme to control the population of monitor lizards, eradicate rats, and remove wild pigs and chickens should ideally be implemented on Loosiep. It should also be noted that since 2005, during the time that fieldwork has been conducted on the islands, turtle take has been prohibited on the five turtle islands by island chiefs. As a result, this project has contributed to the preservation of turtles during the timeframe of yearly fieldwork.
Acknowledgements
This work was funded by the Joint Institute for Marine and Atmospheric Research (JIMAR) and sponsored by the Oceanic Society. The fieldwork would not have been possible without the dedication and hard work of field team leaders, Xavier Maigul and Michael Lingelmar; field assistants, Albert Suwel, Edwin Yatch, Everest Rusumal, John Paul Malisou, Juanito Mangirechog, Max Fasong, and Alexander Sau; and the community of Falalop. A special thanks to Dr Margie Falanruw with Yap Institute of Natural History, Birgit Winning and Wayne Sentman of the Oceanic Society, Yap State Marine Resources Management Division, and Karen Frutchey, Ray Clarke, and Steve Kolinski with JIMAR and NOAA PIRO. For providing metal tags not only to this project, but to tagging programmes across the Pacific, our sincere appreciation to Anne Patricia Trevor with SPREP. Also, thanks to the office of Dr Peter Dutton at the NOAA SFSC, MTRP for assisting with DNA analysis (CITES Permit No. 07US844694/9) and sample vials. My sincere gratitude to Dr Brendan Godley and Dr Annette Broderick with the Marine Turtle Research Group, Centre for Ecology and Conservation, University of Exeter in Cornwall for advising on the project, editing this paper, nominating me for the 2008 British Chelonia Group Scholarship, and for their continued patience and encouragement. Also my appreciation to the British Chelonia Group for providing a scholarship to support the work carried out in Ulithi.
References
Balazs, G.H., & Chaloupka, M. (2004). Thirty-year recovery trend in the once depleted Hawaiian green turtle stock. Biol. Conserv. 117: 491-498.
Bennett, D. (2002). Varanus indicus Mangrove Monitor. In: Little Book of Monitor Lizards. Viper Press, Aberdeen, U.K. p. 61.
Broderick, A.C., Frauenstein, R., Glen, F., Hays, G.C., Jackson, A.L., Pelembe, T., Graeme, G.D., & Godley, B.J. (2006). Are green turtles globally endangered? Global Ecol. and Biogeogr. 15: 21-26.
Buden, D.W. (2007). Reptiles of Satawan Atoll and the Mortlock Islands, Chuuk State, Federated States of Micronesia. Pacific Science 61(3): 415-428.
Chaloupka, M., Bjorndal, K.A., Balazs, G.H., Bolten, A.B., Ehrhart, L.M., Limpus, C.J., Suganuma, H., Troeng, S. & Yamaguchi, M. (2007). Encouraging outlook for recovery of a once severely exploited marine megaherbivore. Global Ecol. and Biogeogr. 17(2): 297-304.
Chaloupka, M. & Limpus, C.J. (2001). Trends in abundance of sea turtles resident in southern Great Barrier Reef waters. Biol. Conserv. 102: 235-249.
Falanruw, M.V.C., McCoy, M.A. & Namlug (1975). Occurrence of Ridley Sea Turtles in the Western Caroline Islands. Microsc 11:1.
Fisher, H. (1948). Locality Records of Pacific Island Reptiles and Amphibians. Copeia 69.
Godley, B.J., Broderick, A.C., Hays, G.C. (2001). Nesting of green turtles (Chelonia mydas) at Ascension Island, South Atlantic. Biol. Conserv. 97: 151-158.
Hays, G.C. (2004). Good news for sea turtles. Trends in Ecol. Evol. 19: 349-351.
Invasive Species Specialist Group (ISSG). Global Invasive Species Database. 9 January 2007. (http://www.issg.org/database/species/ecology.asp?si=1065&fr=1&sts)
Kolinski, S.P., Cruce, J., Parker, D.M., Frutchey, K.P., Balazs, G.H. & Clarke, R. (2007). Identifying Migration-based Connectivity via Satellite Telemetry for Post-nesting Green Turtles from Gielop Island, Yap State, Federated States of Micronesia. MTN (In review).
Kolinski, S.P. (1995). Migrations of the green turtle, Chelonia mydas, breeding in Yap State, Federated States of Micronesia. Microsc. 28(1): 1-8.
Kolinski, S.P. (1992). Outer Islands Turtle Project: Stage II, Report on the Gielop Island Fieldwork. Yap MRMD, unpublished report.
Lessa, W.A. (1983). Sea Turtles and Ritual: Conservation in the Caroline Islands. In: Gunda, B. (Ed) The Fishing Culture of the World, pp 1183-1201.
Lever, C. (2003). Naturalized Reptiles and Amphibians of the World, Oxford Univ Press, 92 pp.
Limpus, C.J., Miller, J.D., Parmenter, C.J. & Limpus, D.J. (2003). The Green Turtle, Chelonia Mydas, Population of Raine Island and The Northern Great Barrier Reef: 1843-2001. Memoirs of the Queensland Museum 49(1): 349-440.
McCoid, M.J. & Wittman, G.J. (1993). Varanus indicus (mangrove monitor) diet. Herp. Rev. 24: 105.
McCoy, M.A. (1981). Subsistence Hunting of Turtles in the Western Pacific: The Caroline Islands. In: Bjorndal, K.A. (Ed) Biology and Conservation of Sea Turtles, pp 275-280. Smithson. Inst. Press, Washington, D.C.
McCoy, M.A. (1974). Man and the Turtle in the Central Carolines. Microsc. 10(2): 207-221.
McGowan, A., Broderick, A.C., Frett, G., Gore, S., Hastings, M., Pickering, A., Wheatley, D., White, J., Witt, M.J., & Godley, B.J. (2008). Down but not out: marine turtles of the British Virgin Islands. Animal Conserv. 11: 92-103.
Richardson, J.I. (1999). Priorities for Studies of Reproduction and Nest Biology. In: Eckert, K.L., Bjorndal, K.A., Abreu-Grobois, F.A. & Donnelly, M. (Eds) Research and Management Techniques for the Conservation of Sea Turtles. IUNC/SSC Marine Turtle Specialists Group Publication No. 4, pp 124-129.
Ruddle, K. (1996). Back to first ‘design principles’: the issue of clearly defined boundaries. SPC Traditional Marine Resource Management and Knowledge Bulletin 6: 4-12.
Solow, A.R., Bjorndal, K.A. & Bolten, A.B. (2002). Annual variation in nesting numbers of marine turtles: the effect of sea surface temperature on re-migration intervals. Ecol. Lett. 5: 742-746.
Troeng, S. & Rankin, E. (2005). Long-term conservation efforts contribute to the positive green turtle Chelonia mydas nesting trend at Tortuguero, Costa Rica. Biol. Conserv. 121: 111-116.
Testudo Volume Seven Number One 2009
Top









