Kylie L. Scales 1, James P. Lewis 2, Rachel T. Graham 2 and Brendan J. Godley 1
1 Marine Turtle Research Group, Centre for Ecology and Conservation, School of Biosciences, University of Exeter, Cornwall Campus, Treliever Road, Penryn, Cornwall TR10 9EZ, UK.
2 Ocean Giants Program, Wildlife Conservation Society, PO Box 76, Punta Gorda, Belize.
Introduction
The Mesoamerican Barrier Reef System (MBRS) is the most extensive in the Northern Hemisphere (Arrivallage & An, 2004), second only in size to the Great Barrier Reef. It is a marine biodiversity hotspot (Roberts et al., 2002) and has been identified as a ‘Global 200’ conservation priority ecoregion (Olson & Dinerstein, 1998). The UNESCO World Heritage Centre (WHC) designated the section of the MBRS that lies within Belizean territorial waters as the Belize Barrier Reef Reserve System (BBRRS) in 1996. This highly productive and diverse system encompasses seven UNESCO World Heritage Sites (UNESCO, 1997-2010), and is of vital ecological and economic importance to the region.
Coral reef ecosystems are in catastrophic decline globally, owing to over-harvesting, pollution, disease and the effects of climate change (Bellwood et al., 2004). Caribbean reefs are amongst the most severely degraded and reef resilience, their propensity for regeneration following extreme events, may be approaching a tipping point beyond which recovery is impossible (Gardner et al., 2003). Belizean reefs have been assessed as over 50% degraded, on a scale from pristine to ecologically extinct (Pandolfi et al., 2003), and are at a critical point in terms of recovery potential (Gardner et al., 2003). As a result of this severe decline, the BBRRS was added to the List of World Heritage in Danger in 2009 (UNESCO, 1997-2010).
Coral reef systems constitute a fundamental habitat in the life cycle of the circum-tropically distributed (van Dam & Diez, 1997) hawksbill turtle. Following the oceanic neonatal stage, juvenile hawksbills (20-25cm straight carapace length, SCL) are recruited to neritic habitats such as coral reefs (Bolten, 2003; Houghton et al., 2003; Blumenthal et al., 2009a). These ecosystems provide juveniles with plentiful resources, necessary to facilitate rapid growth prior to sexual maturity (Bjorndal, 1997). An ontological shift occurs at a certain level of maturity, when hawksbills disperse from the developmental foraging ground, beginning to utilise deeper foraging habitats (Musick & Limpus, 1997; McGowan et al., 2008; Blumenthal, 2009b) and undertaking large-scale migrations of up to 2000km to breeding grounds (van Dam et al., 2008).
Concurrent with the widespread decline of Caribbean coral reefs has been a significant depletion of hawksbill populations in the region. Hawksbills have been exploited for thousands of years (Meylan & Donnelly, 1999), mainly for collection of ‘tortoiseshell’ made from their ornately patterned scutes, but also for eggs and meat (Smith et al., 1992; Witzell, 1994). Other anthropogenically-mediated threats have synergistic deleterious impacts. By-catch (Lewison et al., 2004), pollution (Laist, 1997), coastal development (Bourgeois et al., 2009) and habitat degradation (Gardner et al., 2003) have together caused severe population depletion in the Caribbean region (Meylan, 1999; Troeng et al., 2005). As the largest spongivore, the hawksbill is a keystone species in coral reef ecosystems (Jackson, 1997) and, at historical population density, would have played a vital role in the maintenance of reef resilience. Determination of the status of Caribbean hawksbill populations and their coral reef habitats is essential in the formulation of effective conservation initiatives for both the species itself and the reef systems that it not only depends upon, but actively maintains.
The University of Exeter, in collaboration with the Wildlife Conservation Society (WCS), carried out a preliminary study into the utility of active acoustic telemetry for tracking hawksbills at LRA (Jackson et al., 2010). In order to expand this initial study, we set out to i) determine key biometric parameters of the hawksbill population at Lighthouse Reef Atoll (LRA), Belize, and ii) investigate patterns of abundance across the atoll.
Study Site
This study took place over thirty days, from 24 April to 24 May 2010, at Lighthouse Reef Atoll, Belize. LRA is the most remote of three offshore coral atolls in the BBRS. It is located 75km to the east of Belize City and is approximately 45km long and 10km wide (Fig. 1). Within its coral-rimmed perimeter, LRA encompasses a shallow lagoon containing six small cayes and two ‘no-take’ marine protected areas (MPAs), Blue Hole Natural Monument (BHNM) and Half Moon Caye Natural Monument (HMCNM) (Fig. 1, inset), both of which are UNESCO World Heritage Sites (UNESCO, 1997-2010) and are managed by the Belize Audubon Society (BAS, 2007).
Fig. 1. Map showing geographical location of Lighthouse Reef Atoll.
Methods
Patterns of abundance
A small team of Belizean free-divers was employed to assist with all in-water activities. In order to determine patterns of hawksbill abundance over the atoll as a whole, 49 in-water sightings transects of 1km length were carried out. Starting points for transects were randomly generated within separate habitat zones, to ensure adequate spatial coverage of the entire atoll (Fig. 2). The transect protocol involved a team of three snorkellers swimming southwards parallel to one another at a distance of approximately 10m, and occasionally diving down to facilitate closer inspection of reef features (Fig. 3). Upon sighting a turtle, the snorkeller would raise a hand as a signal. The boat captain would then navigate the boat to the snorkeller’s location, which was recorded using a handheld GPS unit (Garmin GPSMap76). Estimated depth (m) and size (minimum curved carapace length (CCLmin(cm)) were also recorded. 36 of these transects were repeated at least seven days after the initial swim. 13 could not be repeated due to time constraints and inclement weather conditions on the eastern forereef.
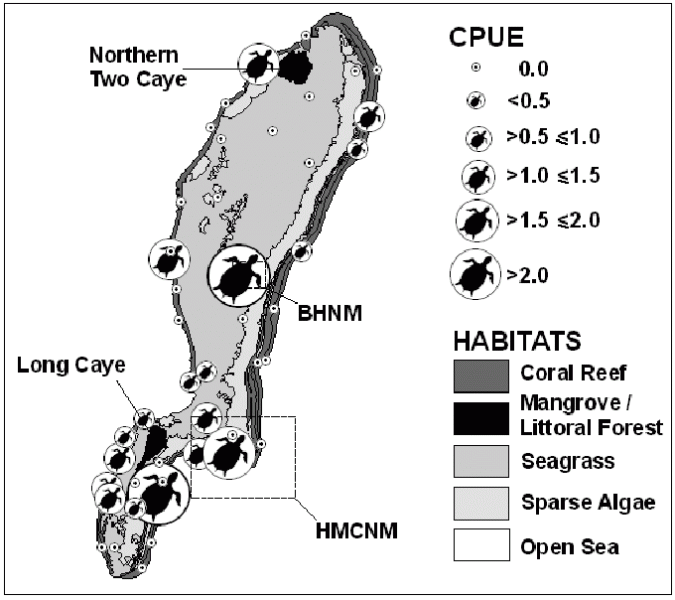
Fig. 2. Turtle abundance (as Catch Per Unit Effort, CPUE), determined by sightings transects. Locations of protected areas (BHNM, HMCNM) also shown. Hawksbills were more numerous in the coral reef habitat than in the lagoon, and within protected areas.

Fig. 3. Three snorkellers conducting a sightings transect within the lagoon.
Turtle capture and measurement
A total of seventeen hawksbills were captured to facilitate the collection of key biometric measurements, and for inter-observer calibration of the estimated sizes recorded during sightings transects. Statistical analysis confirmed that estimates of all observers were within a tolerable range of actual size, which verifies the biometric data from the sightings transects.
Searches for turtles were conducted using teams of snorkellers and focussed on areas that had been identified on the basis of accessibility and greater potential turtle population density. Once a turtle was sighted, estimated size (CCLmin (cm)) and depth (m) and a GPS capture location were recorded. The individual was then captured by our free-diving team (Fig. 4) and brought on board a 23ft skiff. We placed a wet towel over the turtle’s head and kept it in a shaded corner to minimise disturbance (Fig. 5). Additionally, it appeared that gentle massage to the neck of the animal had some calming influence.

Fig. 4. Free-diver capturing a large juvenile hawksbill turtle (CCLmin=59.7cm) within the lagoon habitat.

Fig. 5. Once on board the boat, captured turtles were covered with a wet towel and kept in the shade to minimise discomfort.
We weighed the turtle using a custom-made net and spring balance, into which it was placed on its carapace (Fig. 6). We then took both minimum and maximum curved carapace length (CCLmin; CCLmax: Bolten, 1999), as accurate determination of CCLmin can be operationally difficult in hawksbills. All captured individuals were also marked with Inconel flipper tags in both front flippers (Fig. 7) and released at their capture locations within two hours (Fig. 8).

Fig. 6. Weighing a large juvenile turtle using a custom-made net and spring balance.

Fig. 7. All individuals were tagged with a uniquely numbered Inconel tag in both front flippers, for future identification. Tag positioning allows for growth as the animal matures and minimises opportunity for the tag to chafe on soft tissue.

Fig. 8. Release of a juvenile hawksbill turtle at its capture location.
Results and discussion
Of all hawksbill turtles encountered within this study and that conducted by Jackson (2010) (n=68), 91% were immature (CCLmin=<65cm; Witzell, 1983), suggesting that the atoll is principally utilised by juveniles. LRA comprises approximately 73km2 of suitable hawksbill foraging habitat, much of which is shallow, hence offering protection, shelter and forage for immature individuals. Oceanic current modelling suggests that juvenile hawksbills recruiting to the developmental habitat at LRA could have originated from nesting beaches all over the Caribbean and as far afield as Antigua, Barbados, Venezuela and Puerto Rico (Blumenthal et al., 2009c), although genetic stock analysis (cf. Bowen et al., 2007) would be required to confirm natal origins. As this study population was predominantly juvenile, we conclude that LRA is an important developmental foraging habitat for the regional hawksbill population.
Using ‘catch per unit effort’ (CPUE) calculated from the number of turtles encountered on sightings transects, we determined that hawksbill abundance was appreciably higher in the coral reef habitat than in the lagoon, which may function primarily as a transit zone between foraging zones on the reef. No statistically significant differences in abundance were observed between the eastern and western forereefs, despite considerably more unpredictable abiotic conditions on the eastern forereef resulting from weather patterns.
However, it was only possible to conduct several of the sightings transects on the eastern forereef once, and an unavoidable element of bias may as a result have been introduced to the findings. Repetition of these transects would be necessary to eliminate this potential bias. Further repetition of transects over a greater temporal scale could also elucidate potential seasonal trends and the effects of tropical storms and hurricanes on hawksbill abundance.
CPUE was also found to be significantly higher within the two ‘no-take’ marine protected areas (MPAs), which are closed to fishing and regularly patrolled by the Belize Audubon Society’s (BAS) rangers, than outside their boundaries. We cannot yet be certain as to determining factors of this increased abundance, which may occur simply as a result of the protection of better quality habitat. However, the significantly higher number of turtles encountered in the protected areas than outside their boundaries suggests that complete protection from fishing confers a greater level of resilience to the hawksbill population. Although turtles are not often directly targeted by fisheries at LRA, bycatch in gillnets may be adversely affecting the population. Implementation of gear restrictions such as bans on the use of gillnets could prevent both hawksbill bycatch and overexploitation of the biotic resources at LRA and hence confer protection on both the hawksbill population and the highly diverse ecosystem of which it is an integral part.
Acknowledgements
This study was possible only as a result of substantial input from several individuals and organisations. The financial and logistical support of the Wildlife Conservation Society, British Chelonia Group and Vemco is gratefully acknowledged. Thanks are extended to the Fisheries Department of Belize and the Belize Audubon Society, and also to Sophie Thompson and Alistair Daly for their assistance in data collection and endless patience. The project was only made possible through the extensive efforts of the expedition crew and a debt of gratitude is owed to Dan Castellanos, Daniel Castellanos Sr., Darryn Castellanos, Demian ‘Tobo’ Garbutt, Jason Castro, Mason Cuevas, Alex ‘Clock’ Garbutt, Kevin Castellanos, and Oringtan ‘Tiger’ Burgess.
References
Arrivallage, A. & An, M. (2004). Status of the Coral Reefs of the Mesoamerican Barrier Reef System Project Region, and reefs of El Salvador, Nicaragua and the Pacific Coasts of Mesoamerica. In: Status of Coral Reefs of the World, (Wilkinson, C., ed.), Global Coral Reef Monitoring Network, Townsville: Australian Institute of Marine Sciences. Vol. 2, pp. 473-491.
Belize Audubon Society (BAS) (2007). Management plan; Half Moon Caye Natural Monument and Blue Hole Natural Monument, 2007-2012. Belize City, Belize.
Bellwood, D.R., Hughes, T.P., Folke, C. & Nyström, M. (2004). Confronting the coral reef crisis. Nature 429: 827-833.
Bjorndal, K.A. (1997). Foraging ecology and nutrition of sea turtles. In: The Biology of Sea Turtles (Lutz, P.L. & Musick, J.A., eds), CRC Press, Boca Raton, pp. 199-232.
Blumenthal, J.M., Austin, T.J., Bell, C.D.J., Bothwell, J.B., Broderick, A.C., Ebanks-Petrie, G., Gibb, J.A., Luke, K.E., Olynik, J.R., Orr, M.F., Solomon, J.L. & Godley, B.J. (2009a). Ecology of hawksbill turtles, Eretmochelys imbricata, on a western Caribbean foraging ground. Chel. Cons. Biol. 8: 1-10.
Blumenthal, J.M., Austin, T.J., Bothwell, J.B., Broderick, A.C., Ebanks-Petrie, G., Olynik, J.R., Orr, M.F., Solomon, J.L., Witt, M.J. & Godley, B.J. (2009b). Diving behaviour and movements of juvenile hawksbill turtles Eretmochelys imbricata on a Caribeean coral reef. Coral Reefs 28: 55-65.
Blumenthal, J.M., Abreu-Grobois, F.A., Austin, T.J., Broderick, A.C., Bruford, M.W., Coyne, M.S., Ebanks-Petrie, G., Formia, A., Meylan, P.A., Meylan, A.B. & Godley, B.J. (2009c). Turtle groups or turtle soup: dispersal patterns of hawksbill turtles in the Caribbean. Mol. Ecol. 18: 4841-4853.
Bolten, A.B. (1999). Techniques for measuring sea turtles. In: Research and Management Techniques for the Conservation of Sea Turtles (Eckert, K.L., Bjorndal, K.A., Abreu-Grobois, F.A. & Donnelly, M., eds), IUCN/SSC Marine Turtle Specialist Group Publication No. 4, 1999.
Bolten, A.B. (2003). Variation in sea turtle life history patterns: neritic vs. oceanic developmental stages. In The Biology of Sea Turtles Vol. 2 (Lutz, P.L. et al., eds), CRC Press, Boca Raton, FL, pp. 243-257.
Bourgeois, S., Gilot-Fromont, E., Viallefont, A., Boussamba, F. & Deem, S.L. (2009). Influence of artificial light, logs and erosion on leatherback sea turtle hatchling orientation at Pongara National Park, Gabon. Biol. Conserv. 142: 85-93.
Bowen, B.W., Grant, W.S., Hillis-Starr, Z., Shaver, D.J., Bjorndal, K.A., Bolten, A.B. & Bass, A.L. (2007). Mixed-stock analysis reveals the migrations of juvenile hawksbill turtles (Eretmochelys imbricata) in the Caribbean Sea. Mol. Ecol. 16: 49-60.
Gardner, T.A., Cote, I.M., Gill, J.A., Grant, A. & Watkinson, A.R. (2003). Long-term region-wide declines in Caribbean corals. Science 301: 958-960.
Houghton, J.D.R., Callow, M.J. & Hays, G.C. (2003). Habitat utilization by juvenile hawksbill turtles (Eretmochelys imbricata, Linnaeus, 1766) around a shallow water coral reef. J. Nat. Hist. 37: 1269-1280.
Jackson, J.B.C. (1997). Reefs since Columbus. Coral Reefs 16: 23-32.
Jackson, J., Lewis, J., Graham, R. & Godley, B. (2010). Tracking juvenile hawksbill turtles at Lighthouse Reef Atoll, Belize. Testudo 7(2): 31-42.
Laist, D.W. (1997). Impacts of marine debris: entanglement of marine life in marine debris including a comprehensive list of species with entanglement and ingestion records. In: Marine Debris: Sources, Impacts, Solutions (Coe, J.M. & Rogers, D.B., eds). Springer-Verlag, New York, pp. 99-139.
Lewison, R.L., Crowder, L.B., Read, A.J. & Freeman, S.A. (2004). Understanding impacts of fisheries bycatch on marine megafauna. Trends in Ecology and Evolution 19(11): 598-604.
McGowan, A., Broderick., A.C., Frett, G., Gore, S., Hastings, M., Pickering, A., Wheatley, D., White, J., Witt, M.J. & Godley, B.J. (2008). Down but not out: marine turtles of the British Virgin Islands. Anim. Conserv. 11: 92-103.
Meylan, A.B. (1999). Status of the hawksbill turtle Eretmochelys imbricata in the Caribbean region. Chel. Cons. Biol. 3(2): 177-184.
Meylan, A.B. & Donnelly, M. (1999). Status justification for listing the hawksbill turtle (Eretmochelys imbricata) as critically endangered on the 1996 IUCN Red List of Threatened Animals. Chel. Conserv. Biol. 3(2): 200-224.
Musick, J.A. & Limpus, C.J. (1997). Habitat utilization and migration in juvenile sea turtles. In: Lutz, P.L. & Musick, J.A. (eds). The Biology of Sea Turtles. CRC Press, Boca Raton, Florida.
Olson, D.M. & Dinerstein, E. (1998). A representation approach to conserving the Earth’s most biologically valuable ecoregions. Conservation Biology 12(3): 502-515.
Pandolfi, J.M., Bradbury, R.H., Sala, E., Hughes, T.P., Bjorndal, K.A., Cooke, R.G., McArdle, D., McClenachan, L., Newman, M.J.H., Paredes, G., Warner, R.R. & Jackson, J.B.C. (2003). Global trajectories of the long-term decline of coral reef ecosystems. Science 5635: 955-958.
Roberts, C.M., McClean, C.J., Veron, J.E.N., Hawkins, J.P., Allen, G.R., McAllister, D.E., Mittermeier, C.G., Schueler, F.W., Spalding, M., Wells, F., Vynne, C. & Werner, T.B. (2002). Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 295: 1280-1284.
Smith, G.W., Eckert, K.L. & Gibson, J.P. (1992). WIDECAST Sea Turtle Recovery Action Plan for Belize (Karen L. Eckert, ed.). CEP Technical Report No. 18, UNEP Caribbean Environment Programme, Kingston, Jamaica. 86pp.
Troeng, S., Dutton, P.H. & Evans, D. (2005). Migration of hawksbill turtles Eretmochelys imbricata from Tortuguero, Costa Rica. Ecography 28: 394-402.
United Nations Educational, Scientific and Cultural Organisation (UNESCO) (1997-2010) World Heritage List (online) whc.unesco.org/en/list [Downloaded 11 March 2010].
Van Dam, R.P. & Diez, C.E. (1997). Diving behaviour of immature hawksbill turtles (Eretmochelys imbricata) in a Caribbean reef habitat. Coral Reefs 16: 133-138.
Van Dam, R.P., Diez, C.E., Balazs, G.H., Colón Colón, L.A., McMillan, W.O. & Schroeder, B. (2008). Sex-specific migration patterns of hawksbill turtles breeding at Mona Island, Puerto Rico. Endang. Species Res. 4: 85-94.
Witzell, W.N. (1983). Synopsis of the biological data on the hawksbill turtle Eretmochelys imbricata (Linnaeus, 1766). FAO Fish. Synop. 137: 78.
Witzell, W.N. (1994). The origin, evolution and demise of the U.S. sea turtle fisheries. Mar. Fish. Rev. 56(4): 8-23.
Testudo Volume Seven Number Three.
Top